Chemicals
Mass spectrometry: the technique
5' reading time

Chemicals
5' reading time

Mass spectrometry is one of the most Important analytical techniques used for the determination of trace and ultra-trace elements, due to its high sensitivity and low detection limits (Becker, 2007).
This technique is also used to obtain information on the molecular weight and chemical structures of peptides, proteins, carbohydrates, oligonucleotides, natural products and drug metabolites (Biemann, 2014).
This shows the broad spectrum of application of this technique: from the identification of a specific analyte in the form of traces, to the determination of complex chemical structures with high molecular weight.

Mass spectrometry can be coupled to other analytical techniques such as chromatography (gas and liquid) or with an inductively coupled plasma. This makes it possible to increase the performance of this technique and to obtain both qualitative and quantitative information on the desired analyte.
Mass spectrometry works by ionizing chemical compounds to generate charged molecules or fragments of molecules and then measures their mass-to-charge ratios. Among the main exponents of ionization techniques are John Fenn and Koichi Tanaka. They received the Nobel Prize in Chemistry in 2002, for the development of two soft ionization technologies:
Gentle laser desorption, Dr. Tanaka.
A mass spectrometry analysis consists of ionizing molecules in the gas phase, separating the different ions produced and detecting them.
Below is a diagram that explains how a mass spectrometer works:
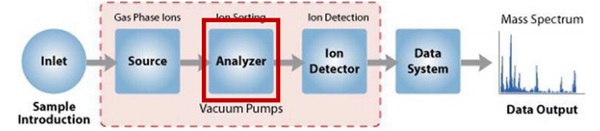
The sample (solid, liquid, gaseous) is ionized..
Ions enter the mass analyzer/filter and are separated based on their mass-to-charge ratio (m/z).
The ions are detected by a mechanism capable of identifying the charged particles (for example an electro multiplier).
Results are displayed as spectra with relative abundance as a function of m/z.
Identification is performed by relating known masses to identified masses or by a characteristic fragmentation pattern.
The arrival of the TANDEM (MS/MS) technique, whose set-up includes two analyzers and a collision chamber, has opened up new analytical horizons, in terms of selectivity of results and reduction of detection limits (LOD).
Below we can see a general overview of the technique :
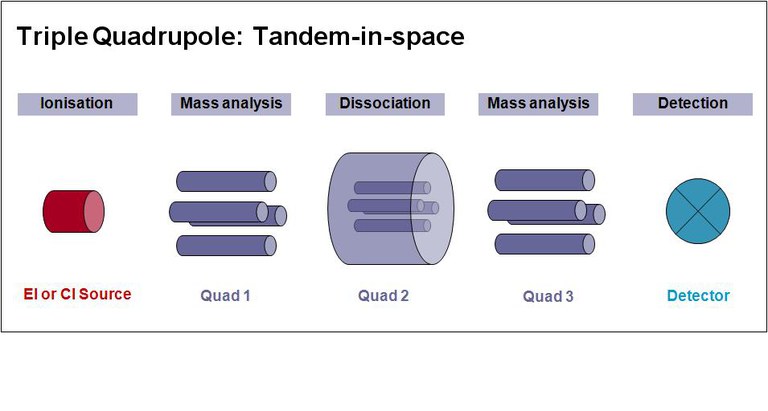
source : www.biologie.hu-berlin.de/de/gruppenseiten/oekologie/meth/massspec/mass_sp
General diagram MS/MS
The first analyzer selects a certain molecular ion (parent ion).
The collision chamber serves to cause the fragmentation of this ion.
The second analyzer measures the mass of the fragments produced.
The choice of solvent:
The correct grade and quality of the solvent are therefore crucial criteria to obtain accurate analytical data. To satisfy the ever-increasing requirements in terms of equipment and detection methods, CARLO ERBA Reagents has developed specific ranges of solvents for UHPLC-MS, LC-MS and GC-MS applications
Our solvents guarantee excellent performance even for the analysis of the most complex mixtures and are tested specifically for each application.
Our quality control laboratories are equipped with state-of-the-art instrumentation that works with a high-efficiency electronic ionization source.To guarantee the quality and technical characteristics of our solvents for gas chromatography (GC-MS line), we carry out specific tests with an electronic ionizer (EI), one of the most widely used ionization methods.
For liquid chromatography solvents (UHPLC-MS and LC-MS lines) we use both an electrospray, mass ionizer also called ESI (Electrospray Ionization) and a diode array spectrophotometric detector (HPLC-DAD) .
Characteristics of our range of solvents for GC-MS :
|
| Discover the range |
Characteristics of our range of solvents for UHPLC-MS:
|  |
| Discover the range |
Characteristics of our range of solvents, additives and mixtures for LC-MS :
|  |
| Discover the range |